Biomedical Calibration
At VIPL Group, we offer precise and reliable biomedical calibration services to ensure that all medical and diagnostic equipment functions accurately and consistently. In healthcare environments, even small measurement errors can compromise patient safety, diagnostic accuracy, and treatment effectiveness.
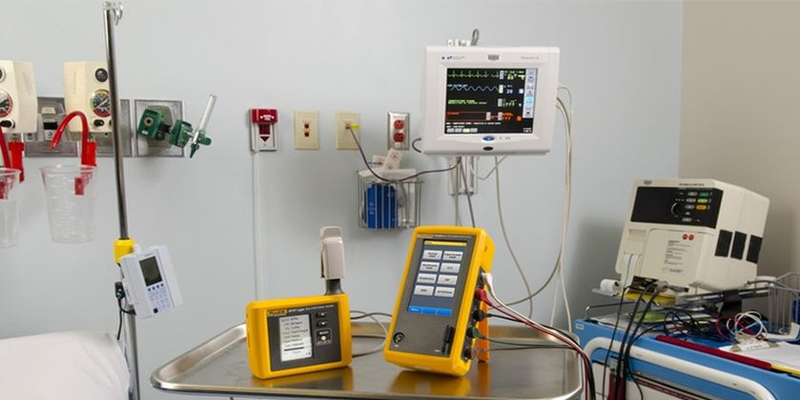
Our calibration solutions help hospitals, diagnostic centers, and research labs maintain equipment accuracy, optimize performance, and comply with national and international standards.
What Is Biomedical Calibration?
Biomedical calibration is the process of verifying, adjusting, and certifying medical devices and laboratory instruments to ensure precise measurements. Over time, frequent use, environmental factors, and wear can cause equipment to drift from its intended performance. VIPL Group follows strict calibration protocols using traceable reference standards to guarantee accurate readings and compliance with healthcare regulations such as ISO, NABL, and IEC standards.
Why Calibration Is Critical in Healthcare
- Accurate Diagnosis & Treatment: Proper calibration ensures reliable readings, supporting accurate clinical decisions and treatment plans.
- Patient Safety: Calibrated equipment reduces the risk of misdiagnosis, incorrect dosing, or harmful interventions.
- Regulatory Compliance: Calibration helps healthcare facilities remain audit-ready and compliant with NABL, ISO, and FDA guidelines.
- Equipment Reliability & Longevity: Routine calibration improves device performance, reduces failures, and extends equipment lifespan.
- Cost Efficiency: Well-calibrated instruments minimize repair costs, reduce downtime, and prevent operational losses.
Biomedical Calibration Services by VIPL Group
We provide comprehensive calibration services for a wide range of biomedical and clinical devices, including:
Patient Monitoring & Diagnostic Equipment
- ECG, EEG, and EMG machines
- BP monitors and pulse oximeters
- Glucometers
- Thermometers and infusion pumps
Laboratory & Analytical Instruments
- Centrifuges and autoclaves
- Spectrophotometers
- Incubators and water baths
- Microscopes and pipettes
Critical Care & Imaging Equipment
- Ventilators
- Defibrillators
- Imaging devices (X-ray, ultrasound, MRI)
- Anesthesia machines
Why Choose VIPL Group for Biomedical Calibration?
- Calibration traceable to national and international standards
- Certified technicians with extensive experience in biomedical devices
- Advanced laboratory with precision reference instruments
- On-site and in-lab calibration support
- Quick turnaround with minimal disruption to hospital operations
- Detailed calibration certificates suitable for audits and regulatory compliance
- Custom calibration schedules for hospitals, clinics, and research labs
Our Calibration Process
Inspection & Pre-Testing: Devices are checked for current performance and potential issues.
Measurement Comparison: Readings are verified against certified reference instruments.
Adjustment & Correction: Deviations are corrected to restore measurement accuracy.
Final Verification: Post-adjustment, devices undergo re-testing to confirm precision.
Certification: Comprehensive certificate includes:
- Measured results
- Uncertainty values
- Traceability details
- Technician and lab information
- Recommended next calibration date
FAQs
Typically every 6–12 months, depending on usage, device type, and industry standards.
Yes, VIPL Group offers both on-site and in-lab calibration services to minimize operational disruption.
We calibrate monitoring, diagnostic, laboratory, imaging, and critical care instruments across hospitals and clinics.
Yes, all certificates are traceable to national and international standards and are audit-ready.
We provide precise, certified, and reliable calibration services backed by trained engineers, advanced equipment, and comprehensive documentation to ensure patient safety and regulatory compliance.

